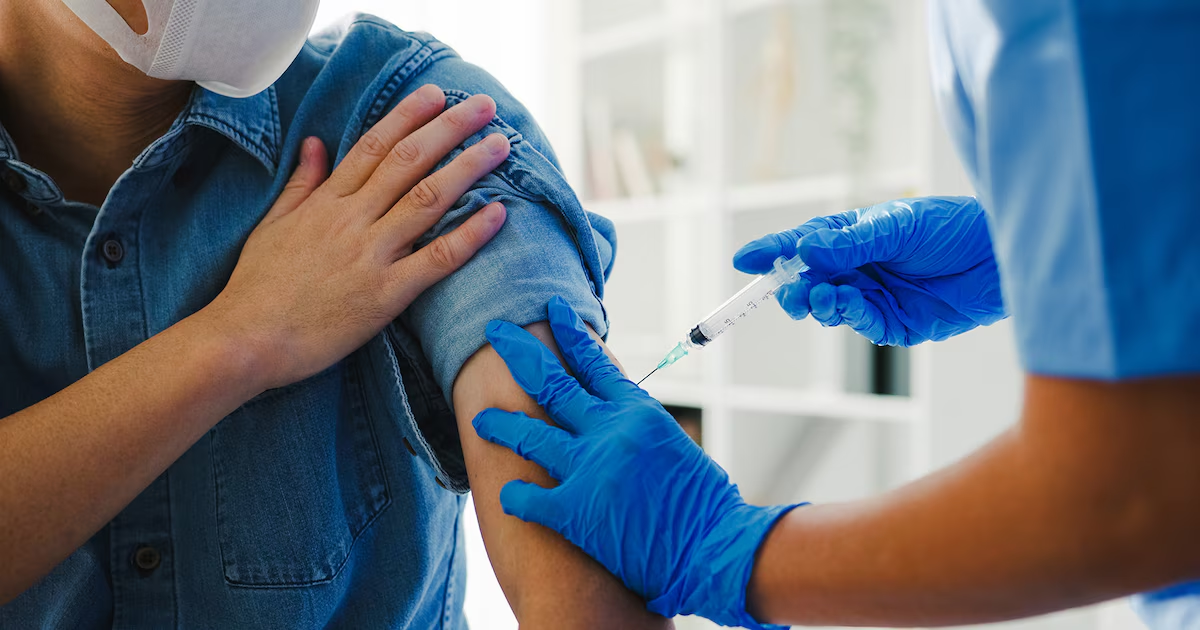

An international clinical trial conducted in the 2022-2023 season found that a new modified messenger RNA (modRNA)-based influenza vaccine demonstrated Higher effectiveness and superior immune responses compared to traditional alternativesaccording to the results published by The New England Journal of Medicine. The study, sponsored by Pfizercontain 18,476 healthy adults between 18 and 64 years old USA, South Africa and the Philippinesand compared the new biologic vaccine with a commonly used inactivated quadrivalent vaccine, FLUZONE (Sanofi Pasteur).
The process included 18,476 volunteers recruited in 242 centers in the United Statesfive inches South Africa and one in Philippines. Participants could not have received a flu vaccine in the past six months. Each volunteer was administered a single intramuscular dose of the experimental modRNA vaccine or the inactivated quadrivalent vaccine FLUZONE. The key objective was to compare the relative effectiveness of both options in preventing laboratory-confirmed influenza episodes with flu-like symptoms at least fourteen days after vaccination.
Efficacy monitoring was extended until May 2023 and safety data were collected until September of that year. The study group reflected the characteristics of the general adult population with a median age of 20 years 43 yearsHe 41.9% men and a 25.1% represent a risk factor for severe flu.

The modRNA vaccine reached a relative efficiency of 34.5% (95% confidence interval 7.4 to 53.9) compared to the inactivated vaccine, based on the 57 confirmed cases of influenza syndrome in the experimental group compared to 87 in the control group. This result met the noninferiority and superiority criteria specified in the study protocol. The intention-to-treat analysis showed similar numbers.
Most episodes were related to strains of Influenza type A (A/H3N2 and A/H1N1), while the incidence of influenza type B cases was minimal during the season analyzed. A supplementary analysis conducted at the end of the season showed a relative effectiveness of 28.7% (95% CI: 0.1 to 49.4). These data demonstrate that the protection provided by the modRNA vaccine exceeds that of the conventional formulation when type A strains predominate.
The experiment evaluated the Immunogenicity by measuring the antibody and T cell response approximately 4,000 participants. The modRNA vaccine promoted higher titers of hemagglutinating antibodies against influenza A strains according to hemagglutination inhibition assays, meeting non-inferiority criteria compared to the control vaccine for these strains. On the other hand, the response against B strains did not reach the non-inferiority parameters, which may be related to the low circulation of these subtypes during the study season.
There is an increase in the cellular reaction Hemagglutinin-specific CD4+ and CD8+ cells higher in the modRNA group, an effect that lasted up to six months after vaccination. For the four included subtypes (two A and two B) more than 70% of those vaccinated achieved seroprotective antibody levels, particularly against the A/H3N2 and A/H1N1 strains.
Safety monitoring included both immediate reactions and adverse events for six months after dosing. He 70.1% of subjects vaccinated with modRNA reported primarily local reactions Pain at the injection sitebefore the 43.1% the control group. Systemic symptoms such as fatigue And Headacheappeared in the 65.8% And 48.7%respectively. The Fever was registered in the 5.6% of the subjects with modRNA and in the 1.7% those who received the conventional vaccine. These reactions were mild or moderate in nature, began two to three days after injection and disappeared within one to two days.
Regarding other adverse events that 6.7% the modRNA group and the 4.9% of the control group reported some incidence up to one month after vaccination, with the majority related to expected reactogenicity. Only one severe case of an injection site reaction and one episode of a grade 4 anaphylactic reaction were counted as possible effects related to the experimental vaccine.
Serious adverse events and deaths (seven in the modRNA group and nine in the control) were found to be unrelated to vaccination. No confirmed cases of Myocarditis, Pericarditisnor relevant differences in serious autoimmune complications between the groups.

The study compared the relative effectiveness with an already approved vaccine, so the results are not comparable to those of a placebo. The low prevalence of B strains limited the ability to assess specific efficacy against these viruses. In addition, the sample included only healthy adults aged 18 to 64 years, so direct data is available Children, immunocompromised people either over 64 years oldthat represent high-risk populations. The process was sponsored and coordinated by Pfizer and is registered in ClinicalTrials.gov at the number NCT05540522.
The use of the modRNA technology It allows for faster vaccine development and better adaptation to circulating strains, avoiding mutation problems associated with traditional methods. The results presented in this phase 3 study suggest that this strategy could represent an important advance in the control of seasonal influenza by providing tools to increase effectiveness and adapt more quickly to the evolution of the virus.


