A group of 34 patients with glioblastoma, the deadliest form of brain cancer, who were treated with an innovative treatment that combines MRI-guided ultrasound techniques along with standard chemotherapy, improved their survival by almost 40%.
This clinical trial, conducted by researchers at the University of Maryland School of Medicine (USA), demonstrates for the first time the survival benefit of using focused ultrasound to open the blood-brain barrier and improve tumor delivery of chemotherapy in patients with brain cancer after surgery.
“Our results are very encouraging. “Using focused ultrasound to open the blood-brain barrier and deliver chemotherapy can significantly increase patient survival,” said study lead researcher Graeme Woodworth. The results are published in The Lancet Oncology.
Glioblastoma is the most common and lethal type of malignant brain tumor. The five-year survival rate is just 5.5%, and patients live an average of 14 to 16 months after diagnosis when treated with surgery, radiotherapy and chemotherapy where appropriate.
One of the major obstacles in treating this cancer is the administration of drugs, which are hindered in their journey to the tumor by the blood-brain barrier, which is a specialized network of blood vessels and brain cells that acts as a safety system for the brain to protect it from the invasion of dangerous toxins and microbes.
the Temozolomide is the standard treatment for glioblastomaBut the drug is usually blocked by the blood-brain barrier. Studies show that less than 20% reaches patients’ brains.
However, this defense can be temporarily opened using a specialized focused ultrasound device. This process begins with the injection of microscopic bubbles filled with an inert gas into the patient’s bloodstream. Under MRI guidance, they target specific areas of the brain as the injected microbubbles rotate.
The trial compared patients with glioblastoma who received focused ultrasound to open the blood-brain barrier before chemotherapy with a similar control group treated with standard temozolomide alone. After surgery, chemotherapy, and radiotherapy, trial participants received up to six monthly sessions of ultrasound plus temozolomide.
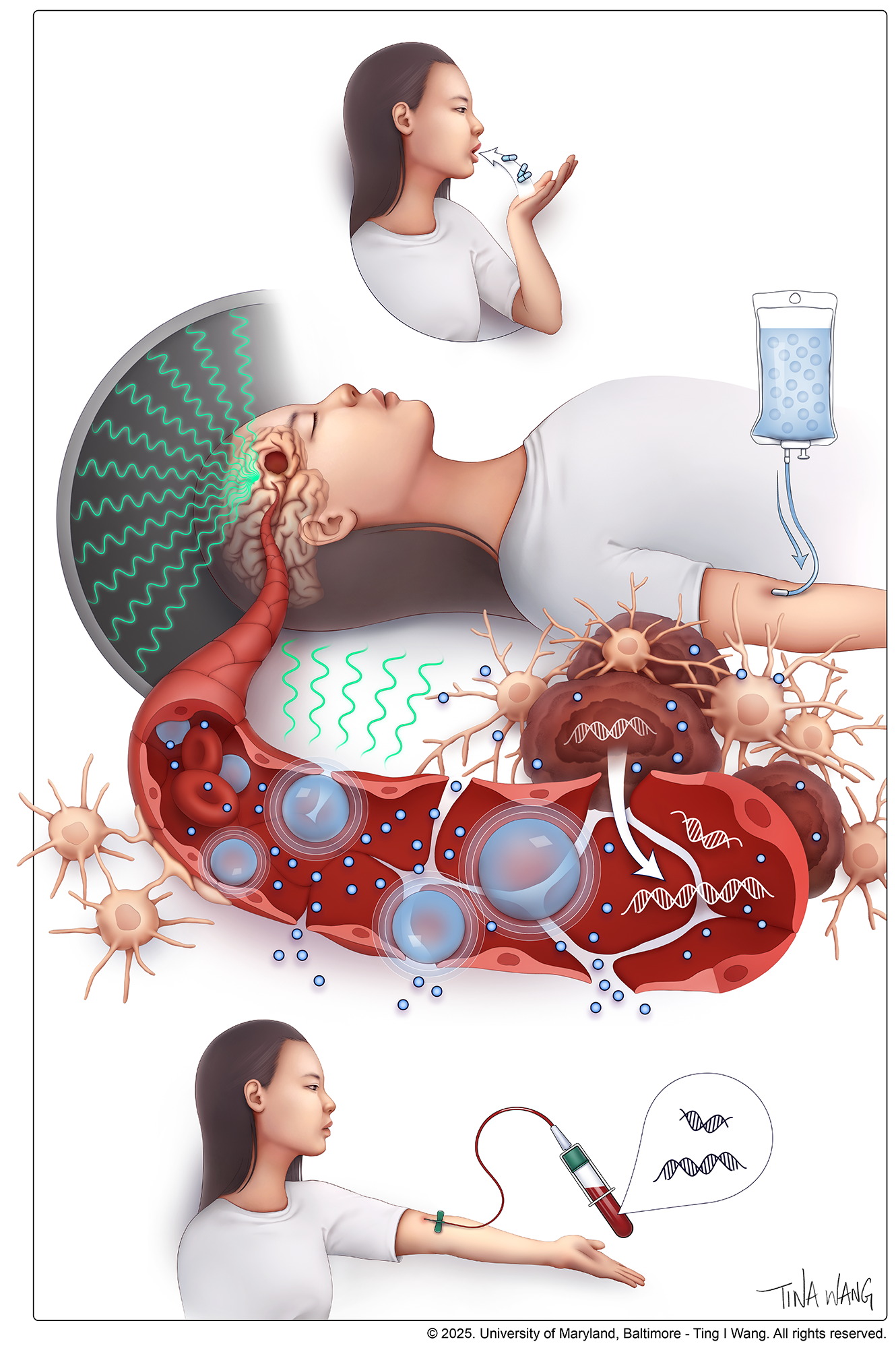
Chart of the new treatment
The researchers explain that releasing biomarkers into the bloodstream provides a new method for simple and routine monitoring of brain regions without the need for invasive biopsies. «The ability to open the blood-brain barrier could also lead to the testing of new treatments “To determine whether it offers greater life-extension benefits,” the researchers say.
The results showed clear benefits: progression-free survival was approximately 14 months (vs. 8 months in the control group) and overall survival exceeded 30 months (vs. 19 months in the control group).
Additionally, research shows that this useful technology allows us to better monitor patients to determine whether brain cancer has developed.
Previous studies by Woodworth have shown that temporary opening of the blood-brain barrier can be performed safely and feasibly in patients with brain tumors.